One of the most tangible ways artificial intelligence is already reshaping healthcare is through medical imaging
Over the past decade, MRI, CT, and ultrasound technologies have progressed dramatically, generating images of unprecedented quality and detail. But richer images also mean vastly more data. Interpreting billions of pixels at scale, consistently and accurately, remains a challenge no human can meet alone. That is where AI comes in.
By last year (2025), AI-enabled imaging solutions had become a $2.4 billion global market, driven largely by early disease detection and real-time clinical assistance. According to Global Growth Insights, the market should reach $26.5 billion by 2035.
From oncology to cardiology, algorithms are increasingly being used not to replace clinicians, but to augment them - spotting subtle anomalies, ensuring exam completeness, and reducing the margin for error.
In prenatal medicine, where timing can change a life, those gains are particularly critical.
Enter BrightHeart.

Turning complex fetal heart screening into a routine exam
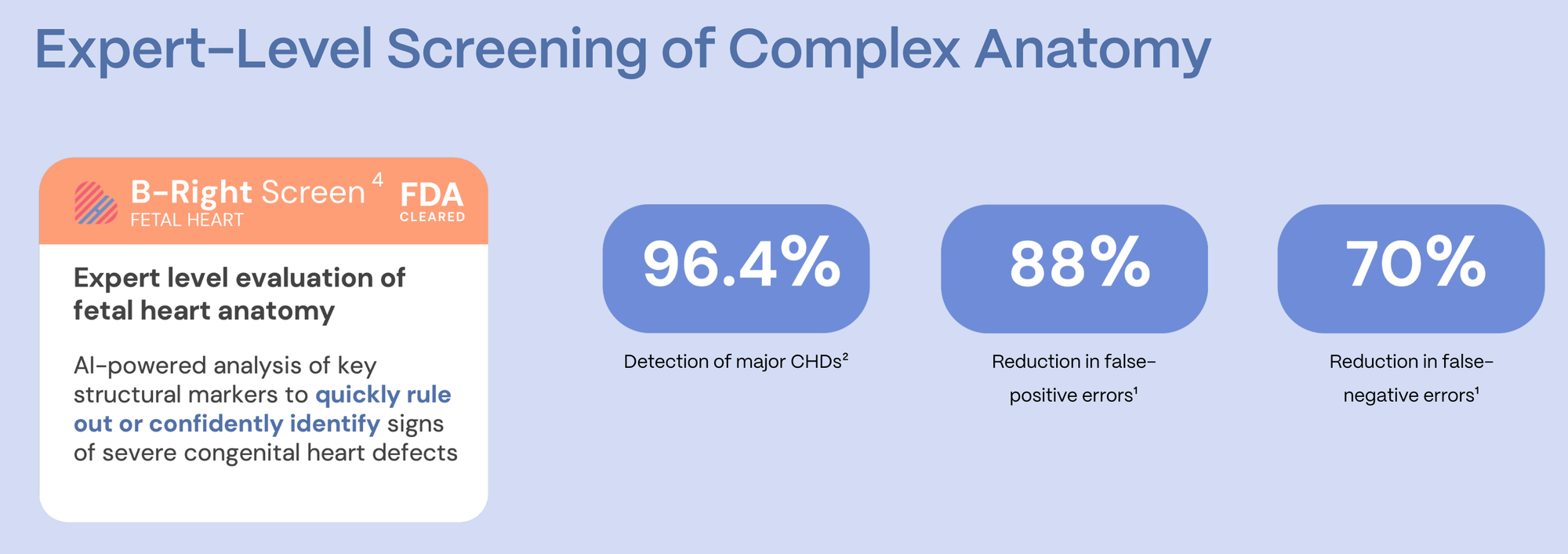
BrightHeart is an AI-powered medical device designed to support gynecologists, obstetricians, and sonographers during prenatal ultrasound exams. Its focus is on one of the most complex and most frequently missed areas of prenatal screening: congenital heart defects (CHD).
Founded in 2022 within Sofinnova Partners’ MD Startup Studio, BrightHeart was built at the intersection of clinical expertise and deep-tech execution. Cécile Dupont, CEO of BrightHeart and Partner at Sofinnova Partners, led the company. Her background combines engineering and medical training.
“Detecting cardiac anomalies in utero is extremely difficult for clinicians who are not pediatric cardiologists,” Dupont said. “At three months of pregnancy, the fetal heart is about one centimeter in size, and historically, only around one third of cardiac anomalies are detected.”
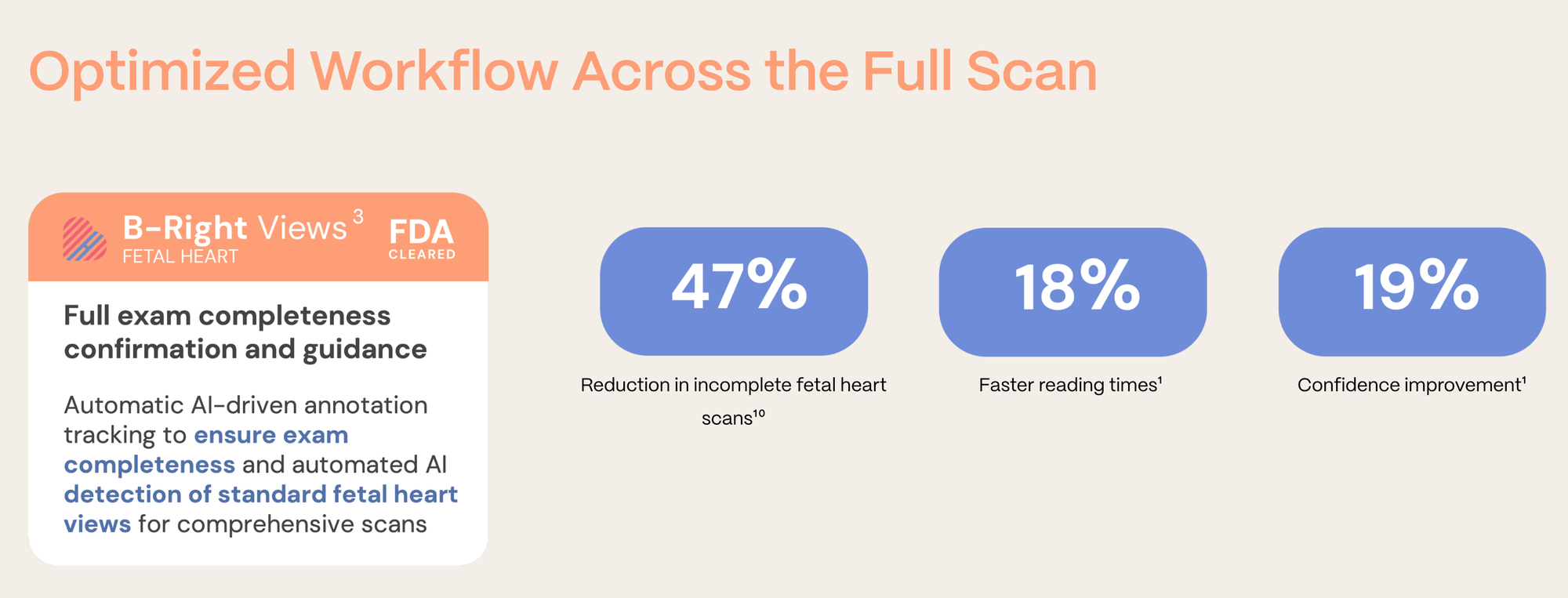
BrightHeart’s workflow solution uses AI to guide clinicians through the ultrasound exam, helping them capture the right views, validate exam completeness, and flag potential abnormalities without disrupting existing workflows.
The impact is significant: fetal cardiac anomaly detection rates by those using the solution have improved from roughly 30% to over 90%, and in some settings exceeded 96%.
A clinical problem hiding in plain sight
The idea behind BrightHeart originated at Necker Hospital in Paris, one of Europe’s leading pediatric centers. Pediatric cardiologists Marilyne Lévy and Bertrand Stos witnessed firsthand the consequences of missed prenatal diagnoses.
Around 70% of congenital heart defects are not detected during pregnancy, even though they affect approximately 3% of births, making them more common than chromosomal conditions such as Down syndrome. While the fetus initially benefits from the mother’s circulation, the condition is often discovered only after birth, when oxygen deprivation can already have caused irreversible damage.
“These babies arrive without warning,” Dupont said. “If severe heart disease is detected too late, the consequences can be devastating.”
The clinicians believed artificial intelligence could help close this diagnostic gap and approached Sofinnova with the concept. BrightHeart was created to translate the clinical reasoning of expert cardiologists into an intelligent algorithm capable of interpreting highly complex ultrasound images.
The original clinicians remained involved as advisors, while continuing their hospital practice.
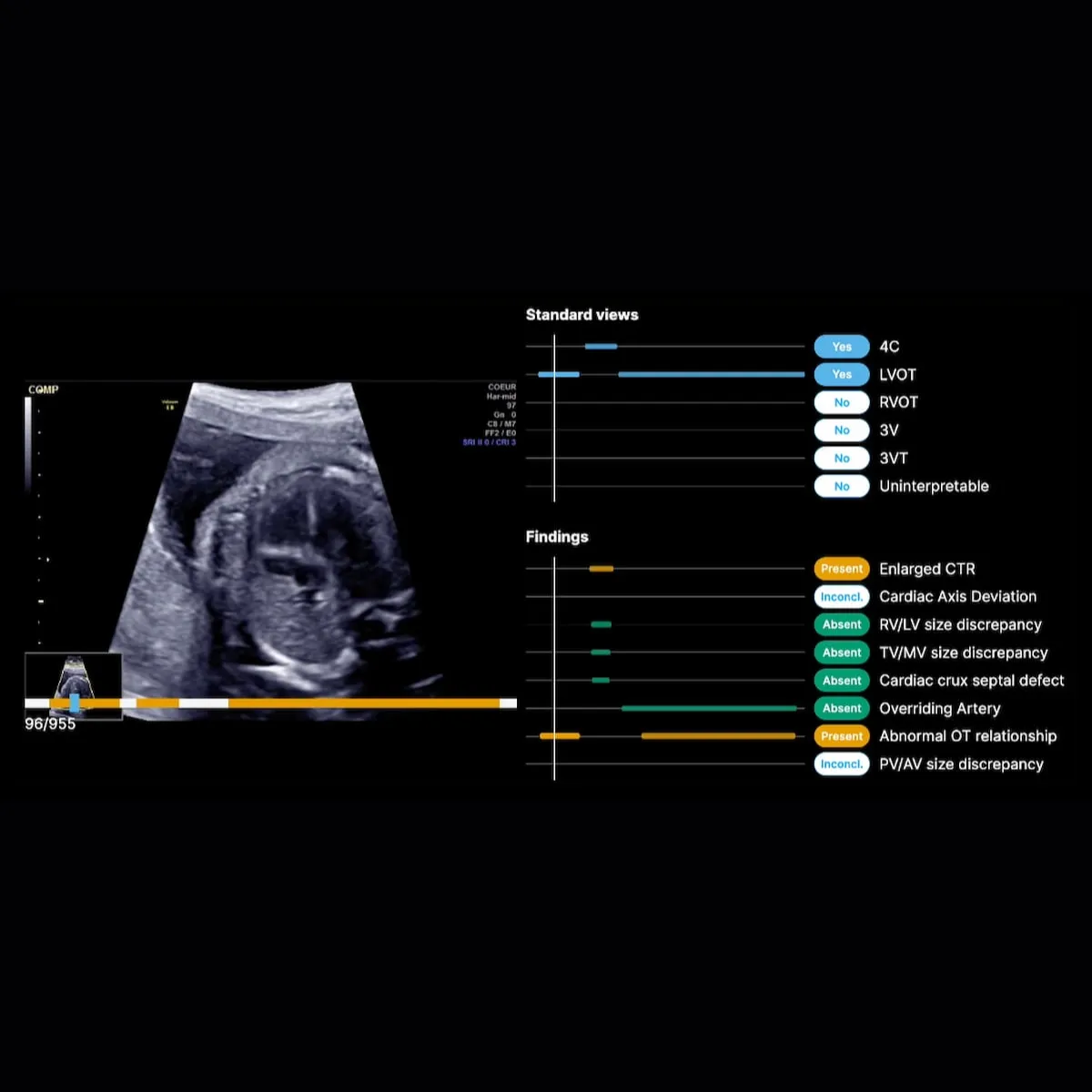
A tablet, a checklist, and eight critical markers
BrightHeart’s technology is designed to fit seamlessly into existing clinical environments. The system relied on a tablet placed next to the ultrasound machine, connected securely to the cloud. During the exam, the software guides the sonographer through a structured checklist, ensuring all required views are captured.
“The system doesn’t allow you to stop until the exam is complete,” Dupont explained.
The AI analyzes eight key pathological markers, using a color-coded system to indicate whether findings are normal, abnormal, or require further attention. This not only improved detection, but also reduced the need to recall patients due to incomplete exams - a major operational and emotional burden in prenatal care.
Importantly, BrightHeart does not provide a diagnosis. It functions as a clinical decision-support tool. If warning signs appear, patients are referred to pediatric cardiologists for definitive diagnosis and care planning, including decisions about delivery in specialized Level 3 maternity units when needed.
The next version of the software is expected to operate in real time. “The tablet was a technical choice to standardize deployment and maintenance,” Dupont said. “But the solution itself is hardware-agnostic.”
From regulatory hurdles to real-world impact
As a regulated medical device, BrightHeart faces long development and validation timelines, including technical and clinical validation for FDA clearance.
Like many French AI imaging startups, the company has chosen to expand first into the United States, where regulatory pathways are more predictable. Between early 2024 and mid-2025, BrightHeart began commercial deployment.
One of its earliest adopters is Carnegie in New York, the city’s largest obstetric ultrasound center. After one year of use, the results were striking: exam completion rates improved by 34%, liability risks were reduced, and exam time was shortened by at least five minutes. That efficiency gain compounded significantly across daily workflows.
In Europe, however, regulatory timelines are more challenging. The introduction of the Medical Device Regulation (MDR) has required all devices certified under the old CE mark to be re-certified, causing delays across the sector.
“The process is extremely frustrating,” Dupont said. “But we are nearing approval.”
Once obtained, the company planned to prioritize France while evaluating other European markets where it could deliver the most impact.

Building the standard of care, not just another tool
BrightHeart’s ambition is not incremental improvement, but standardization.
The company aims to make expert-level fetal heart screening as routine as Down syndrome screening is today. Its cloud-based platform covers the full workflow - from image acquisition to reporting - and is built on four pillars: clinical accuracy, workflow optimization, seamless integration with all ultrasound machines, and strong peer-reviewed clinical evidence.
So far, BrightHeart is the only company in its category with published peer-reviewed clinical results in leading obstetrical journals.
The long-term roadmap extended beyond the heart. “We started with the most complex organ,” Dupont said. “But our goal is to expand to all critical organs and make the prenatal exam both complete and faster.”
A subscription model with measurable ROI
BrightHeart operates on a monthly subscription model, bundling both the tablet and the software. Pricing was not publicly disclosed due to competitive sensitivity, but the value proposition was clear.
By saving time, improving exam quality, and reducing recall rates, the platform allowed clinicians to increase throughput - even in healthcare systems without direct reimbursement for AI tools. In practice, the solution positively impacts both workflow efficiency and practitioner income.
While competition in AI imaging is intensifying, BrightHeart remains uniquely focused on screening rather than diagnosis.
“It’s becoming a very hot space,” Dupont said. “But the clinical need is real, and the bar for evidence is high.”
€11 million to scale globally
BrightHeart has just announced the close of an €11 million Series A round co-led by Odyssée Venture and GO Capital, with participation from the Mussallem CHD Alliance, Lift Value, IDAHO HealthTech Club via Side Angels, Sofinnova Partners, and numerous clinicians and medtech entrepreneurs.
The funding was earmarked for product expansion, U.S. and European growth, and continued clinical validation. At the time, the company employed 10 people across France and the United States, spanning R&D, clinical affairs, and sales.
“Our mission is to make AI the new standard of care in prenatal ultrasound,” Dupont said. “If we can detect severe heart disease before birth, we can change outcomes for babies and families at scale.”